The Science behind Scent and the Art of Perfume Making
In the previous article, we discussed how perfume notes and chords are composed by a multitude of molecules. How are these molecules discovered and made? How can we determine what is inside a perfume product? The role of chemistry, especially organic synthesis and analytical methods, in addressing these aspects is addressed below.
How are fragrances extracted and made?
Fragrances are often labelled with natural aroma, like “rose”, “lavender”, “citrus” or “musk”. Does it mean that perfume oils are always extracted from flowers, fruits, and animals?
It is no surprise that perfume oils were historically extracted from natural plant and animal sources.1, 2 The simplest method of extraction is expression, such as pressing orange peels to extract citrus oils. Nevertheless, not all fragrances can be extracted in such a straightforward manner.
The isolation of fragrant molecules can be aided by heat. In steam distillation, steam is passed through the natural material and helps drive the evaporation and co-distillation of fragrant molecules. After condensation to liquid phase, the oil, known as essential oil, is obtained as a layer separate from the water layer due to their different densities.
Given their hydrophobic nature (see Figure 1 of previous article), many fragrant molecules can be extracted from water by solvent extraction. According to the principle “like dissolves like”, it is more favourable for hydrophobic molecules to be dissolved in the organic, hydrophobic solvent than water. In ancient Egypt, a technique called enfleurage was employed to extract fragrances from plants. The plant, such as jasmine, is pressed onto a layer of fat for months, to allow molecules to adsorb to the fat without the risk of heat degradation. Afterwards, the molecules are extracted from the fat with ethanol. In hot enfleurage or maceration, heated oil is used instead of fat. In modern times, petroleum-based organic solvents such as petroleum ether and hexane are more commonly used.
So far extraction from nature seems very feasible. What if the material is difficult to obtain, such as musk from musk deer? What if the plant only grows in certain seasons or in certain places? In the past, the time required for plant cultivation, and the costs of cultivation and extraction from natural sources, meant that perfumes were expensive and only used by the very upper class. Introduction of synthetic molecules into perfumes, first of coumarin in Fougère Royale (1882) and later of aliphatic aldehydes in Chanel No 5 (1921), has not only lowered the cost of perfume oil extraction, but also expanded the toolkit available to perfumers who can trial novel molecules and combinations.1-3 Sometimes molecules that look alike to natural products are synthesised.4 These synthetic analogues can help perfumers discover molecules with improved properties such as smell, volatility, or stability, and also help them understand how molecular shape may influence odour, commonly known as structure-activity relationship. A perfumer, sometimes referred to as a nose in the industry, can create the desired aroma by combinations of natural and synthetic molecules. Chemical analysis (see next section) of the atmosphere in an odorous environment, called headspace technology, may aid the design of such compositions.5
How are perfumes analysed for quality and safety?
Perfume is a highly complex mixture of molecules unique to the product. The exact chemical composition is considered a trade secret, so in many legislations full labelling of the specific ingredients is not required, with “fragrance” or “perfume” often sufficient. In that case, how can counterfeit perfumes be identified? How can contaminants or possible allergens or carcinogens be detected? The EU requires labelling of possible allergens, such as coumarin, eugenol, geraniol, linalool, and limonene, when their concentrations exceed 0.001%.
A sample of perfume is too complex a mixture to be analysed in one go. Firstly, the many molecules need to be separated. In analytical chemistry, a common separation technique is chromatography. You may have used it to analyse a food dye, where its components are brought up by a solvent to different levels on a paper strip and thus separated. This works because each species interacts with the paper and the solvent with different affinities. Species interacting less favourably with paper or more favourably with solvent will move further up the strip.
For volatile organic compounds, gas chromatography (GC) is often used. Here, an inert carrier gas such as helium, instead of a liquid solvent, is used to move the molecules injected from the perfume sample. Along the continuous flow, depending on physical properties such as volatility, each species requires a different amount of time to pass through a long capillary column, which is tens of meters long and is analogous to the paper strip. Each species exits the column at different time points, thus allowing separation.
Secondly, the separated chemical species needs to be identified. In a gas chromatography-mass spectrometry (GC-MS) system (Figure 1), based on the principle that heavier molecules move more slowly than lighter ones, the molecular masses of the exiting species are measured. Comparison of the time measured by chromatography and mass spectrum enables us to determine the composition of the sample and the concentration of each species.
In the GC-MS analysis of perfumes (Figure 2), one can directly inject a diluted liquid sample to determine its entire content, including less volatile fixative molecules. Alternatively, the air above the perfume sample, known as the headspace, can be introduced into the GC-MS system, thus mimicking the actual scent arising from perfume evaporation. The application of GC-MS has enabled efficient yet rigorous quality control, testing of contaminants and allergens, and perfume content analysis, both by the perfume company itself but also its competitors.

Figure 1: GC-MS instrument at the Department of Chemistry, HKBU.
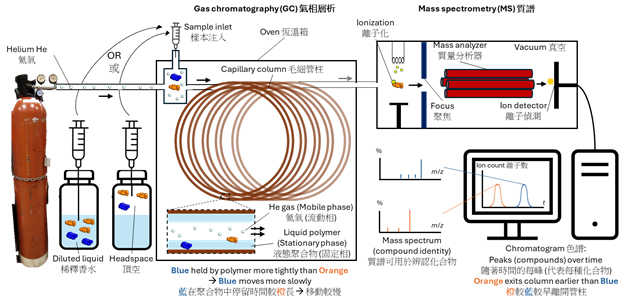
Figure 2: Illustration of GC-MS working principles.
Outlook
As seen above, advances in organic and analytical chemistry have helped us know more about molecules that generate aroma. Can we now predict what a novel molecule would smell like given its structure? Can we now know and recreate the chemical composition of a perfume needed to exactly simulate a certain setting, such as the smell in a floral garden?
Physiological studies, including those of Nobel laureates (2004) Richard Axel and Linda Buck, have informed us that odour comes from the combined responses of hundreds of G protein-coupled receptors (GPCRs) in our nose, activated by the binding of possibly tens to hundreds of thousands of chemicals. The shape and functional groups of a molecule certainly relate to its odour, but as fragrance chemist Philip Kraft (2009) said, “It’s tough work and despite all the molecular design tools, you still have to be lucky”.4 There are still a lot of mysteries about our own olfaction. The design and combination of molecules to create an appealing scent remain largely empirical.
To help select the best molecules, can we now make machines that mimic our olfactory system? One possibility comes from the development of optoelectronic noses.6 They have been shown to enable fingerprinting of different perfumes by producing unique arrays of colours on a 24-element plate. Each element on the plate responds by performing a specific type of reaction with a perfume ingredient.
While perfumery will likely remain as a combination of art and science, progresses in perfumery and chemistry will remain complementary. As Ernest Beaux, the maker of Chanel No. 5, said, “In perfumery the future lies primarily in the hands of the chemists”.1
References
- Fortineau, A.-D. Chemistry Perfumes Your Daily Life. J. Chem. Educ. 2004, 81 (1), 45. DOI: 10.1021/ed081p45.
- Logan, J. L.; Rumbaugh, C. E. The Chemistry of Perfume: A Laboratory Course for Nonscience Majors. J. Chem. Educ. 2012, 89 (5), 613-619. DOI: 10.1021/ed2004033.
- David, O. R. P.; Doro, F. Industrial Fragrance Chemistry: A Brief Historical Perspective. Eur. J. Org. Chem. 2023, 26 (44), e202300900. DOI: 10.1002/ejoc.202300900.
- Davies, E. The sweet scent of success. Chemistry World 2009, February 2009,
https://www.chemistryworld.com/features/the-sweet-scent-of-success/3004856.article - Lear, S. Perfumery: the molecular art form. Chemistry World 2015, October 2015,
https://www.chemistryworld.com/features/perfumery-the-molecular-art-form/9003.article - Sun, L.; Zhang, R.; Hu, L.; Chen, X.; Lu, X.; Li, Z. Hydrophobic and Rapid-Response Sensor Inks: Array-Based Fingerprinting of Perfumes. ACS Appl. Mater. Interfaces 2022, 14 (23), 27339-27346. DOI: 10.1021/acsami.2c03081.
By Dr. Chan H. T.