How the Fragrance of your Perfume is more than any Spice
Those who first encounter organic chemistry are introduced to two major classes of compounds – aliphatic compounds and aromatic compounds. The latter are so named because of the aroma characterising the earlier identified compounds belonging to the class. While not all aromatic compounds smell nice, and not all fragrant molecules are aromatic, the characterisation of substances based on their ability to stimulate our sense of smell has been well known over millennia. Indeed, the earliest chemist found in historical records was Tapputi-Belatekallim, a female perfume maker in Mesopotamia in around 1200 BC. In Cyprus, archaeologists have found evidence of perfume production at least 4000 years ago.
Over the course of history, the arts and sciences of perfumery and chemistry have been developing synergistically.1, 2 The perfume industry, with its global appeal, high profit margins, and multi-billion-dollar market value, has prompted advances in chemistry, particularly in the understanding of natural products and fragrant chemicals, and in the development of organic synthesis and analytical methods, as discussed in the following.
What is in a perfume?
While a bottle of perfume might appear as one uniform liquid, it is by no means simple! A typical perfume may consist of 100-2000 compounds. Perfumes are classified according to the concentration of perfume oil, with the most concentrated being parfum, followed by eau de parfum, eau de toilette, and eau de cologne, all of them being in an ethanol-water solvent.
Solvent
The major solvent in perfume is ethanol (typically >70%) mixed with water. As you may remember from the ethanol-based hand sanitizers used during COVID-19, an ethanol-water mixture has a lower boiling point than water and is volatile, meaning that it evaporates easily and does not leave our skin wet. In perfume, evaporation often is aided by spraying through an atomizer, where small liquid droplets have high contact surface area with air that facilitates solvent evaporation. The antiseptic nature of ethanol helps prevent perfume from microbial degradation. Furthermore, unlike water, ethanol can better dissolve the hydrophobic organic molecules that are described below.
Perfume oil
Fragrance arises from the perfume oil, which contains molecules both of natural and synthetic origins. A variety of functional groups can be found in these molecules (Figure 1). 3, 4.
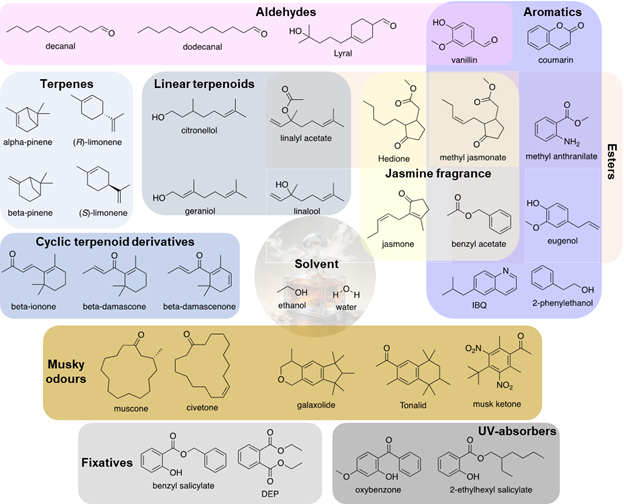
Figure 1: Examples of perfume ingredients.
Terpenes are a class of natural products consisting of carbon and hydrogen in the formula (C5H8)n, where n ≥ 2, with C=C double bonds. Cyclic terpenes are found in perfumes, such as pinene, as both α- and β-isomers. The isomers have the same molecular formula C10H16, but differ structurally by the location of C=C double bond. Another example is (R)-limonene obtained from citrus fruits, also with the molecular formula C10H16, and thus an isomer of pinene. Note that the (S)-isomer of limonene, with the alternative orientation of the isopropenyl group, has a pine-like odour. Oxygen-containing derivatives of terpenes, also called terpenoids, give rise to various fragrances. Examples include the linear terpenoids citronellol, geraniol (both in rose), linalool and linalyl acetate (in lavender), and the cyclic terpenoid derivatives β-ionone, β-damascone, and β-damascenone (all in rose).
Linalyl acetate consists of an ester group, which is often found in fragrant molecules. Examples in perfumes include benzyl acetate and methyl jasmonate (both in jasmine), and the hydrogenated derivative of the latter, Hedione ((+)-cis-methyl dihydrojasmonate). Cis-jasmone, a closely structurally related natural product from jasmine, is also used.
Aldehyde is another commonly found functional group. The aromatic aldehyde vanillin (in vanilla beans), a series of aliphatic linear-chain aldehydes such as decanal (with 10 carbons) and dodecanal (with 12 carbons) which are key to creating Chanel No 5, and the cyclohexenyl-containing synthetic molecule Lyral (lily-of-the-valley odour) exemplify the diverse odours of aldehydes.
As the name implies, aromatic compounds are often present in fragrances. Examples include coumarin (in tonka beans, with its availability from synthesis enabling Houbigant’s Fougère Royale), eugenol (in clove), 2-phenylethanol (in rose), methyl anthranilate (smell of grapes), and 6-isobutyl quinoline (IBQ, smell of leather). Tonalid, galaxolide, and musk ketone all have musky odours that enrich the palette available to perfumers.
Natural sources of musky odours include muscone and civetone, both of which are macrocyclic ketones with respective ring sizes of 15 and 17. The structure and syntheses of these macrocycles and their analogues were studied extensively by Leopold Ružička, who was awarded the Nobel Prize in Chemistry in 1939 “for his work on polymethylenes and higher terpenes”. His research had received significant support and interests from perfume manufacturers.
Notes and Chords
Upon spraying, perfume droplets are dispersed onto our skin. Due to heat from our skin, molecules in the perfume gradually evaporate. As the origin of the name “perfume” in Latin, per fume (“through smoke”), implies, the molecules diffuse in air and enter our noses, creating an overall aroma.1 The gradual evaporation of molecules, dictated by their respective volatility, can be described as “notes” in analogy to music. The top (or head) notes are perceived first and contributed by the most volatile molecules, such as the linear-chain aldehydes. Later, the middle (or heart) notes become apparent, contributed by less volatile molecules like β-ionone and hydrogen bond-forming alcohols including geraniol, citronellol and 2-phenylethanol. As the top and middle notes dissipate, the base notes are perceived last, coming from heavier, less volatile molecules like muscone and civetone. Note that above certain concentrations, some compounds may start to smell unpleasant to us. Hence, the concentration of each ingredient is meticulously formulated by perfumers to compose an overall harmonious, pleasant “chord” of aroma.
Fixative
To sustain the scent from top-note molecules, fixatives which interact favourably with these volatile molecules are often added to delay their evaporation. Some base notes, such as civetone, muscone, and galaxolide, act as fixatives. Other fixatives include benzyl salicylate and the odourless solvent diethyl phthalate (DEP). Compared to ethanol, they both have low volatility and are greasier and more hydrophobic, enabling them to effectively solvate the volatile top-note molecules and slow their evaporation. The evaporation of aldehydes may further be slowed by possible formation of acetals with the ethanol solvent, or imines with nitrogen nucleophiles such as methyl anthranilate (Figure 2). 5
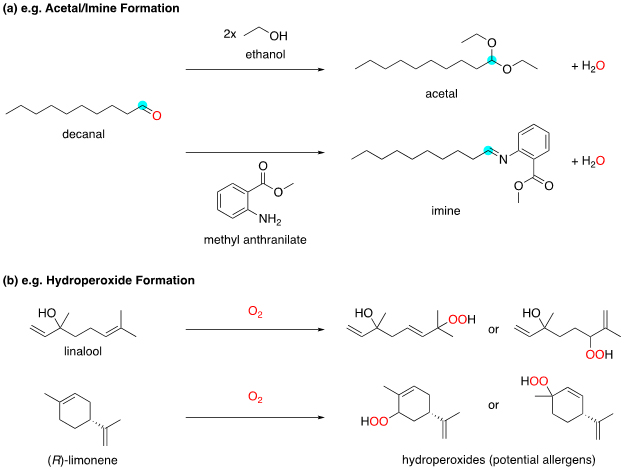
Figure 2: Examples of reactions involving perfume ingredients.
Additives
While perfumes are usually stored in closed bottles and not exposed to air until use, additives are often added to enhance the stability of molecules. For example, to prevent limonene and linalool from oxidation by oxygen in air into hydroperoxides (Figure 2), which may cause contact allergic reactions, antioxidants may be added. UV-absorbers such as oxybenzone and 2-ethylhexyl salicylate are added to reduce damage to molecules caused by energy from exposure to sunlight.
Summary
The above list of molecules found in perfumes is by no means exhaustive, and as perfumery continues to develop, the list will only become longer over time. How are these molecules made and mixed to create a new perfume? How can the content of a perfume be analysed? Seeing that there is so much to take in already this time, these questions will be discussed in the next article.
References
- Fortineau, A.-D. Chemistry Perfumes Your Daily Life. J. Chem. Educ. 2004, 81 (1), 45. DOI: 10.1021/ed081p45.
- Logan, J. L.; Rumbaugh, C. E. The Chemistry of Perfume: A Laboratory Course for Nonscience Majors. J. Chem. Educ. 2012, 89 (5), 613-619. DOI: 10.1021/ed2004033.
- David, O. R. P.; Doro, F. Industrial Fragrance Chemistry: A Brief Historical Perspective. Eur. J. Org. Chem. 2023, 26 (44), e202300900. DOI: 10.1002/ejoc.202300900.
- Davies, E. The sweet scent of success. Chemistry World 2009, February 2009,
https://www.chemistryworld.com/features/the-sweet-scent-of-success/3004856.article - Lear, S. Perfumery: the molecular art form. Chemistry World 2015, October 2015,
https://www.chemistryworld.com/features/perfumery-the-molecular-art-form/9003.article
By Dr. Chan H. T.