Gelatinization: The Secret of Pearls' Texture
Hong Kong's summer weather is hot, making a cup of icy bubble tea a popular choice for many to beat the heat while walking around. This has led to a rise in demand for cool drinks. Beverage shops are everywhere, offering a wide range of options like hand-crushed lemon tea, taro milk, and mango smoothies. The most well-known of these is bubble tea. The key feature of bubble tea is the "pearls," which refer to the tapioca balls used as an ingredient. Made from natural tapioca starch, these pearls become chewy when cooked. Starch is a common component in our diets, present in foods like white rice, corn, and pumpkin (figure 1). Yet, despite both being starchy, pearls are chewier than cooked white rice. Why do pearls end up chewier than white rice after cooking?

Figure 1: Foods that are rich in starch.
All of this is closely related to the chemical structure of starch. To understand the difference in texture between pearls and white rice, we first need to explore the chemical process of cooking starch. Starch is composed of long-chain polysaccharide molecules, which are divided into two main types: amylose and amylopectin. Amylose is a straight-chain starch that formed by α1–4 glycosidic bonds, while amylopectin is a branched-chain starch that includes both α1–4 and α1–6 glycosidic bonds (figure 2).1 The structure of amylose is more regular, forming a helical arrangement, while amylopectin has a branched, tree-like structure, making it more irregular. When we cook white rice or tapioca pearls, the usual process involves adding water followed by heat, triggering a chemical reaction in cooking known as gelatinization. Starch molecules are originally tightly connected by intermolecular hydrogen bonds, forming a compact structure. This structure makes it difficult for water molecules to penetrate, so the starch is insoluble in water at room temperature. However, during gelatinization, heat disrupts the crystalline regions within the starch, creating gaps between the tightly packed molecules and loosening the structure, allowing water molecules to penetrate and form hydrogen bonds with the starch molecules. The absorption and swelling of starch are known as swelling, transforming into a paste-like state.2 If the starch absorbs more water, the molecules can disperse further and become completely surrounded by water, resulting in the formation of a semi-transparent gel. By the way, the common cooking technique of thickening sauces is also based on the gelatinization of starch.
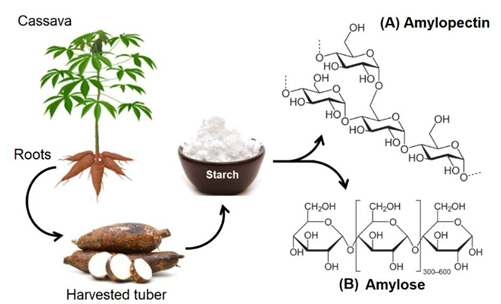
Figure 2: “Illustration of the cassava plant, its tuber, and the appearance of the starch that is extracted from them. In addition, the structures of its main biopolymeric components (amylose and amylopectin) are shown.” by Palencia, M.,García-Quintero, A.,Combatt, E. M. is licensed under CC BY-NC-SA 4.0
The unique textures of white rice and tapioca pearls arise from the different proportions of starch they contain. The ratio of straight-chain amylose to branched-chain amylopectin plays a crucial role in the texture of food after cooking. Because amylopectin has a more branched and irregular structure, it absorbs water and forms a gel easier than amylose. Amylose, with its tight helical arrangement, is less prone to water absorption than amylopectin (figure 3). This difference becomes particularly evident during heating, leading to a significant variation in the texture of the cooked food: white rice tends to be firmer, while tapioca pearls are softer and more glutinous. Specifically, foods with a higher amylose content, such as certain types of rice, have a firmer and drier texture after cooking, maintaining a more distinct graininess. In contrast, foods with a higher amylopectin content, like glutinous rice, become soft, sticky, and stretchy when cooked. Taking Hong Kong's common Jasmine rice as an example, its amylopectin content is about 72 to 78%,4 while glutinous rice can have up to 99% amylopectin content,5 which explains why glutinous rice is softer and more glutinous than Jasmine rice. As for tapioca pearls, they are made from tapioca starch, which contains about 82% amylopectin,6 making them softer and more glutinous after cooking compared to Jasmine rice. Besides the starch ratio, the texture of tapioca pearls is also affected by the degree of starch gelatinization during cooking. The level of gelatinization determines the elasticity and chewiness of the pearls. Insufficient boiling may leave them too hard, while excessive boiling could make them too soft and glutinous, losing the desired texture.
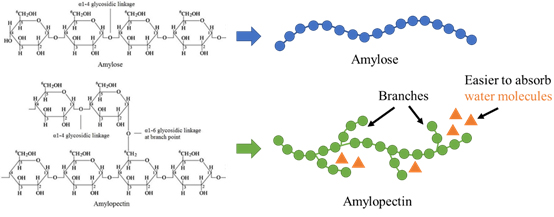
Figure 3: This work, “Influence of amylose and amylopectin structure during gelatinization”, is adapted from “Structure of amylose and amylopectin in starch.” by Nawaz, H., Waheed, R., Nawaz, M., Shahwar, D., used under CC BY 3.0
Although pearls are a popular ingredient in many bubble tea drinks, they are primarily made from tapioca and do not provide other nutrients such as protein, vitamins, or minerals. Tapioca itself is a high-calorie ingredient with no flavor. Therefore, sugar is added during the cooking process of the pearls which further increases their caloric content. A 2019 report by the Consumer Council pointed out that many bubble tea drinks have very high sugar content and caloric values.7 The sugar in pearl milk tea comes not only from the added sugar and creamer but also from the pearls themselves, which are a significant source of sugar. On average, more than a quarter of the sugar content in a serving of pearl milk tea comes from the pearls. Other ingredients, such as syrup, concentrated fruit juice, cheese, and taro, also increase the total sugar content and caloric value of the drink. Therefore, bubble tea drinks are a hidden source of high sugar intake. Excessive sugar consumption can lead to health problems related to obesity, diabetes, and cardiovascular diseases. So it's better to consume these drinks less!
References
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and Chemical Modifications in Starch Structure and Reactivity. IntechOpen, 2020. DOI: 10.5772/intechopen.88870
- Jiménez, A.; Fabra, M. J. ; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review, Food Bioprocess Technol, 2012, 5, 20582076.
- Palencia, M.; García-Quintero, A. ;Combatt, E. M. Biopolymers Containing in Cassava Starch (Manihot Esculenta): Amylose and Amylopectin. J. Sci. Technol. Appl., 2025, 19, Art-110, 1-9.
- Srikaeo, K.; Sopade, P. A. Carbohydr. Functional Properties and Starch Digestibility of Instant Jasmine Rice Porridges. Carbohydr. Polym., 2010, 82 (3), 952-957.
- Rini; Yenrina, R.; Anggraini, T. ; Chania, N. E. The Effects of Various Way of Processing Black Glutinous Rice (Oryza sativa L. Processing Var Glutinosa) on Digestibility and Energy Value of the Products. IOP Conf. Ser.: Earth Environ. Sci., 2019, 327, 012013.
- Research News. Healthier tapioca starch is on the way. RIKEN, 2021. https://www.riken.jp/en/news_pubs/research_news/pr/2021/20211217_1/index.html
- Consumer Council, 2019. https://www.consumer.org.hk/tc/article/508-tea-drinks
By Dr. Yim K. H.